Antibiotics are one of the most precious resources in modern medicine. Yet, the fear of a post-antibiotic era is looming over the scientific community. Discovery of the mcr-1 gene within Escherichia coli caused international alarm last November since it confers resistance to colistin – often referred to as the ‘last resort’ antibiotic. The spread of this gene could have disastrous consequences due to the possibility of transfer to more virulent bacteria via independent loops of DNA, known as plasmids.
However, the concept of continuous evolution towards drug-resistant bacterial populations is nothing new. 1928 saw the discovery of penicillin, in which Oxford’s Dunn School of Pathology played an instrumental role. It was Sir Alexander Fleming, in his Nobel Prize Lecture, who reminded us that ‘exposing microbes to non-lethal quantities of the drug make them resistant’. Although resistance is initially triggered by random mutations within bacteria, the reliability of human antibiotic use is ultimately responsible for the extent of the problem – what can be done to resolve this public health burden?
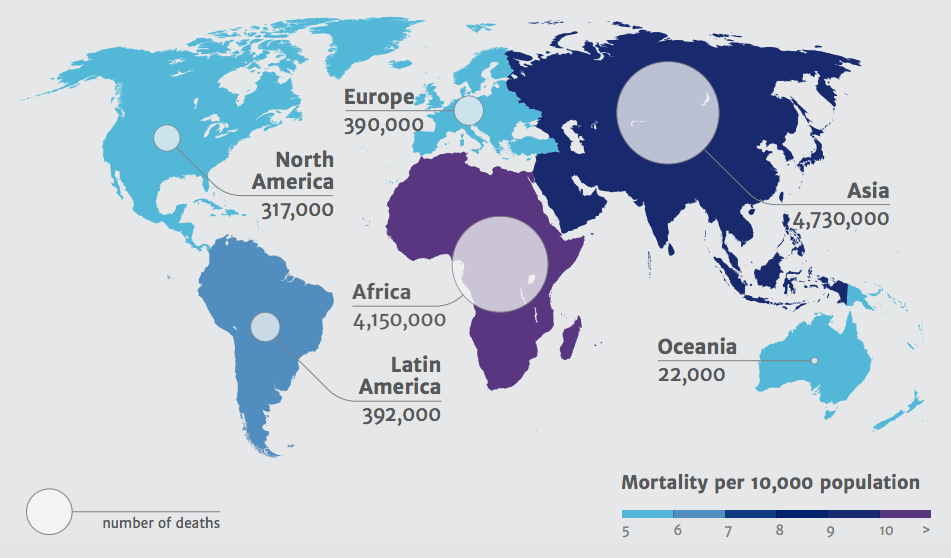
Deaths attributable to antimicrobial resistance by 2050; Source: Review on Antimicrobial Resistance 2014
Resistance to antibiotics currently results in an estimated 700,000 deaths per year, with this figure expected to rise to 10 million by 2050. Lack of effective antibiotics would not only make minor bacterial infections untreatable, but simple operations such as hip replacements and caesarean sections could become life-threatening. Despite becoming increasingly widespread, antimicrobial resistance is still largely misunderstood by the general public. The Wellcome Trust commissioned a study to investigate attitudes towards antibiotics, and discovered many people believe that it is the patients who build up a resistance to antibiotics, rather the causative bacteria. Such findings prompted discussions on whether tackling the public’s misunderstanding is as important as tackling the drug resistance itself – since patient behaviour, including not completing a course of antibiotics, can create a breeding ground for resistant bacteria. Suggestions to move away from the term ‘antimicrobial resistance’ towards ‘drug-resistant infections’, and efforts to replicate the same validation patients feel when prescribed antibiotics with a non-antibiotic prescription, would hopefully work to heighten public awareness of the resistance threat.
Barriers to fighting antibiotic resistance include the challenges of protecting existing drugs, and difficulties in developing novel ones. A new infectious disease has been discovered almost annually in the last 20 years, yet no new antibiotics have been developed in this time – a phenomenon named the ‘discovery void’. The process of drug development is lengthy, yet upon licensure new antibiotics should still be sold and used sparingly to prevent resistance re-emerging. This ‘antimicrobial stewardship’ is a healthcare system approach aiming to encourage prudent use to preserve future effectiveness of antibiotics, however as a commercial model offers few research incentives. Along with the shorter treatment courses compared to chronic conditions, this leads to pharmaceutical companies perceiving antibiotic research as financially unattractive. January’s World Economic Forum in Davos, Switzerland, saw over 80 pharmaceutical and biotech companies signing the ‘Declaration on Combating Antimicrobial Resistance’ – aiming to overcome the unfavourable market of antibiotic development through government collaboration. Suggested incentives – including reimbursement and extension of patent lives – should hopefully reinvigorate this area of drug development and encourage laboratories across the globe to fight resistance.
So when is it time to truly worry about drug-resistant infections? A Nature article in December 2015 suggested that colistin resistance represents a substantial threat to healthcare provision. This, however, ‘does not spell bacterial apocalypse – yet’, since other antibiotics or a larger dose could overcome multi-drug resistant bacteria. Also highlighted was the need to focus on alternative public health interventions, such as vaccines and improved diagnostics – since bacteria will inevitably become resistant to any new class of antibiotics.
Overall, infection rates of the most famous ‘superbug’, methicillin-resistant Staphylococcus aureus (MRSA), saw an 85% reduction between 2003 and 2011 – this success proves that with the support of politicians and policymakers, we could strive to control other drug-resistant infections. This global issue requires a global solution, and a surveillance system should be implemented through international collaboration – allowing the control of bacterial evolution.
![Antibiotic Resistance – A Global Problem Needing Global Solutions Antibiotics are one of the most precious resources in modern medicine. Yet, the fear of a post-antibiotic era is looming over the scientific community. Discovery […]](/wp-content/uploads/2016/01/1.-Bacteria-620x300.jpg)